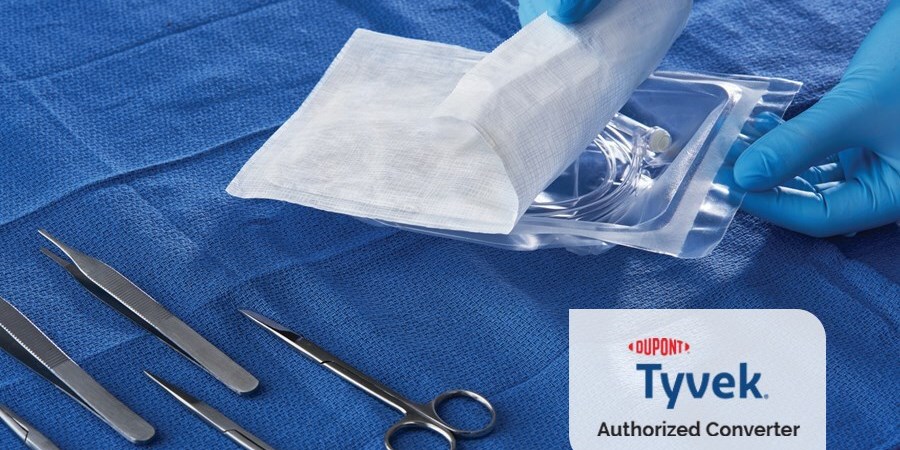
Spectrum provides pouches, header bags, and lids made with DuPont™ Tyvek®, providing the highest level of protection. We are one of the few authorized DuPont™ Tyvek® converters in the world for the healthcare industry.

Spectrum offers a wide variety of single-layer and co-extruded high-performance films that interface well with automation for maximum yields and manufacturing efficiencies in your manufacturing sites converting applications.

Our converted sterile pouches and header bags are puncture resistant and are customized to suit your sterilization and material requirements.

Medical devices or components are placed in a permeable peel pouch made with Tyvek®. The permeability of Tyvek® allows for the complete sterilization of the product while providing an outstanding microbial barrier for the safe handling of the product inside.

Spectrum header bags feature strong linear low-density polyethylene with coated Tyvek® headers. They are puncture- and moisture-resistant and ideal for EO sterilization.

Spectrum offers coated lids CT1073, CT1059, and CT2FS. These products can be printed, unprinted. die-cut or square-cut. Respectively CT1073, CT1059, and CT2FS are made from DuPont™ Tyvek® 1073B, 1059B, and 2FS providing the highest level of protection for your most demanding applications.


