


Injection Molding
Our injection-molded solutions include premium, high-quality components and fully assembled medical devices. We have years of experience with complex molding projects working with highly engineered performance medical plastics.

Multishot injection molding requires expertise in material interactions and understanding of how to combine multiple materials in one mold. We have the capability to support complex components from concept to production.
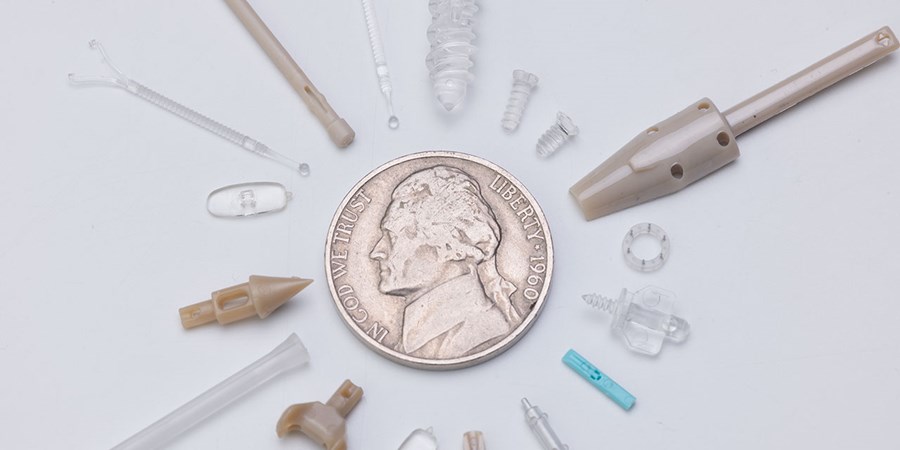
As medical treatments become less invasive, medical devices are getting smaller. We have the expertise to do precision micromolding, including specialized equipment, tool designs, and measurement systems.

We have decades of experience molding onto metal and other substrates. Combining properties of different materials, such as strength and flexibility, can create more intricate designs for aesthetics, branding, or ergonomics.

Implantable and bioresorbable materials pose unique preparation, processing, and handling challenges. As a pioneer with these materials, we have expertise and dedicated resources for manufacturing implantable and bioresorbable plastic components.

Our silicone molding capabilities include liquid injection molding as well as silicone rubber transfer molding, overmolding, and liquid injection molding.

As a single-source partner, we can support your complex surgical device throughout the product lifecycle, from design for manufacturability to development to validation and manufacturing.

Injection mold prototyping is the process of developing a tool to expedite a molded part. DuPont™ Spectrum™ can create your part as close to the intended product design and functionality as possible using your requirements.
Polyetheretherketone (PEEK) is a high-strength, high-temperature thermoplastic with superior mechanical and chemical properties ideal for injection molding applications.

A next generation shaping process, micro-MIM quickly, consistently and cost-effectively produces tiny medical device parts and features with tight tolerances at high volumes.
Medical injection molding for medical device industry requires specialized expertise beyond conventional plastic manufacturing capabilities. The process demands stringent quality management systems (ISO 13485:2016 certified) with comprehensive documentation and traceability throughout production. Cleanroom environments (typically Class 7 or 8) prevent contamination, while validated processes ensure reproducibility across production runs. Material selection involves medical-grade resins with appropriate biocompatibility certifications for patient contact. Precision tolerances often exceed general industry standards, typically achieving ±0.001" or better for critical dimensions. These requirements collectively ensure components meet the exacting standards required for medical devices subject to regulatory scrutiny.
Regulatory compliance for medical injection molding begins with comprehensive documentation and validation. Design history files maintain complete records from concept to production, while material certifications document biocompatibility testing (ISO 10993, USP Class VI) for patient-contact components. Process validation following IQ/OQ/PQ protocols ensures manufacturing consistency, with statistical process control monitoring critical parameters throughout production. Rigorous change control procedures prevent unauthorized modifications that could impact regulatory status. Finished device testing verifies performance specifications, and full traceability from raw material to finished component supports regulatory submissions while enabling targeted recalls if necessary.
Material selection for medical injection molding requires systematic evaluation of application-specific requirements. Biocompatibility testing requirements must align with intended patient contact duration and type, while sterilization method compatibility (EtO, autoclave, gamma) influences material stability. Mechanical performance needs include strength, flexibility, impact resistance, and wear characteristics appropriate for the device function. Chemical resistance against bodily fluids, cleaning agents, and medications prevents premature degradation. Regulatory history and master file availability streamline submission processes, while material cost and processing characteristics impact manufacturing economics. These considerations collectively determine the optimal resin for specific medical applications.
Micromolding technology enables the production of increasingly miniaturized components essential for minimally invasive medical procedures. Specialized injection units provide precise shot control for components weighing less than 1 gram, while micro-detailed tool fabrication achieves feature sizes as small as 0.0015". Advanced vision systems perform 100% inspection of microscopic features impossible to verify with conventional methods. Specialized material formulations optimize flow characteristics for micro-features without compromising mechanical properties. These capabilities collectively enable sophisticated medical devices that reduce surgical trauma, accelerate patient recovery, and enable procedures in previously inaccessible anatomical locations.
We’re happy to help with your projects in any way we can. Contact us and we’ll email you back the information you’re looking for, or we’ll schedule a call to discuss with you in more detail.

DuPont™ Spectrum™ is the starting point for medical device development requiring critical components. Our off-the-shelf tubing and components are available in our Component Webstore to accelerate the design and development of your next project.

